Treatment of autoimmune hemolytic anemias
Treatment of autoimmune hemolytic anemias
Abstract
Autoimmune hemolytic anemia (AIHA) is a relatively uncommon disorder caused by autoantibodies directed against self red blood cells. It can be idiopathic or secondary, and classified as warm, cold (cold hemagglutinin disease (CAD) and paroxysmal cold hemoglobinuria) or mixed, according to the thermal range of the autoantibody. AIHA may develop gradually, or have a fulminant onset with life-threatening anemia. The treatment of AIHA is still not evidence-based. The first-line therapy for warm AIHA are corticosteroids, which are effective in 70–85% of patients and should be slowly tapered over a time period of 6–12 months. For refractory/relapsed cases, the current sequence of second-line therapy is splenectomy (effective approx. in 2 out of 3 cases but with a presumed cure rate of up to 20%), rituximab (effective in approx. 80–90% of cases), and thereafter any of the immunosuppressive drugs (azathioprine, cyclophosphamide, cyclosporin, mycophenolate mofetil). Additional therapies are intravenous immunoglobulins, danazol, plasma-exchange, and alemtuzumab and high-dose cyclophosphamide as last resort option. As the experience with rituximab evolves, it is likely that this drug will be located at an earlier point in therapy of warm AIHA, before more toxic immunosuppressants, and in place of splenectomy in some cases. In CAD, rituximab is now recommended as first-line treatment.
Introduction
Autoimmune hemolytic anemia (AIHA) is a relatively uncommon disorder caused by autoantibodies directed against self red blood cells, with an estimated incidence in adults of 0.8–3 per 105/year, a prevalence of 17:100,000 and a mortality rate of 11%.1,2 It can be idiopathic (50%) or secondary to lymphoproliferative syndromes (20%), autoimmune diseases (20%), infections and tumors.3 AIHA is very rare in infancy and childhood (0.2 per 105/year),4 where it is primary in 37% and associated with immune disorders in 53% of cases. Mortality is lower in children (4%), but rises to 10% if the hemolytic anemia is associated with immune thrombocytopenia (Evans syndrome).5 AIHA is classified as warm, cold (which includes cold hemagglutinin disease (CAD) and paroxysmal cold hemoglobinuria) or mixed, according to the thermal range of the autoantibody. The diagnosis is usually simple, based on the presence of hemolytic anemia and serological evidence of anti-erythrocyte antibodies, detectable by the direct antiglobulin test (DAT). In warm AIHA, DAT is typically positive with anti-IgG antisera (and anti C3d in some cases). Cold forms are usually due to IgM, and the DAT is positive for C3d, since IgM antibodies are often lost or only present in small amounts on the red blood cells at 37°C. It is important to remember that DAT may yield false-negative results due to IgA autoantibodies (that are not detectable by most routine reagents), low-affinity IgG, or RBC-bound IgG below the threshold of the test. For the former two conditions, the use of mono-specific antisera against IgA and low ionic strength solutions or cold washings can overcome the DAT negativity. Small amounts of RBC-bound IgG can be detected employing techniques that are more sensitive than the traditional DAT-tube, such as microcolumn, solid-phase, enzyme-linked, and flow cytometry. Finally, there are rare cases of warm AIHA caused by IgM ‘warm’ autoantibodies that may require special tests (dual DAT) for diagnosis, and are characterized by more severe hemolysis and more fatalities than other types of AIHA. Despite the numerous tests available, approximately 10% of AIHA remain DAT negative, and the diagnosis is made after exclusion of other causes of hemolysis and on the basis of the clinical response to therapy. These atypical cases, which are identified with increasing frequency, may represent a critical diagnostic problem and cause delays in therapy.1,6,7
AIHA may develop gradually, with concomitant physiological compensation, or may have a fulminant onset with profound, life-threatening anemia. Clinical features are determined by the presence/absence of underlying diseases and co-morbidities, and by the rate and type of hemolysis that mainly depends on the characteristics of the autoantibody. In particular, IgM warm AIHA often have more severe hemolysis and more fatalities (up to 22%) than patients with other types of AIHA.6 It is worth remembering that the degree of anemia also depends on the efficacy of the erythroblastic response. In fact, patients with reticulocytopenia, reported to occur in some 20% of adults8 and 39% of children,5 may need very strong transfusion support and represent a clinical emergency.9 The treatment of AIHA is still not evidence-based as there is only one randomized study10 and few prospective phase II trials.11–15 We will briefly consider the main therapeutic tools for this disease, with a focus on patients with idiopathic AIHA refractory to the traditional therapy.
Treatment of warm AIHA
The traditional treatment of AIHA includes corticosteroids, splenectomy and conventional immunosuppressive drugs. Over recent years, some new therapies have become available and there has been some evidence of success. These therapies are primarily used in patients who are not candidates for or fail to respond to splenectomy, those who relapse after splenectomy, and those who cannot maintain stable hemoglobin levels without unacceptably high doses of corticosteroids.
First-line therapy
Corticosteroids
There is general agreement that corticosteroids represent the first-line treatment for patients with warm antibody type AIHA, albeit their use is based on experience rather than hard evidence. In fact, there is little published information on their effectiveness,1,16,17 and this is not supported by clinical trials. Corticosteroids, usually prednisone, are given at the initial dose of 1.0–1.5 mg/kg/day for 1–3 weeks until hemoglobin levels greater than 10 g/dL are reached. Response occurs mainly during the second week, and if none or minimal improvement is observed in the third week, this therapy is assumed to be ineffective. After stabilization of hemoglobin, prednisone should be gradually and slowly tapered off at 10–15 mg weekly to a daily dose of 20–30 mg, then by 5 mg every 1–2 weeks until a dose of 15 mg, and subsequently by 2.5 mg every two weeks with the aim of withdrawing the drug. Although one might be tempted to discontinue steroids more rapidly, AIHA patients should be treated for a minimum of three or four months with low doses of prednisone (≤10 mg/day).1 In fact, patients receiving low doses of corticosteroids for more than six months have a lower incidence of relapse and longer duration of remission than those discontinuing the medication within six months.18 Moreover, an earlier onset of steroid therapy correlates with a lower probability of relapse.16 It is worth remembering that AIHA patients on prolonged steroid therapy should be given bisphosphonates, vitamin D, calcium, and folic acid supplementation.2 Patients with particularly rapid hemolysis and very severe anemia, or complex cases such as Evans syndrome, may require intravenous methylprednisolone at 100–200 mg/day for 10–14 days or 250–1000 mg/day for 1–3 days, although high-dose corticosteroid therapy for AIHA has been described essentially as case reports.19,20 First-line therapy with corticosteroids is expected to provide a response in 70–85% of patients; however, only 1 in 3 cases remain in long-term remission once the drug is discontinued, a further 50% require maintenance doses, and approximately 20–30% need additional second-line therapies. It is not known how many adult patients are cured by steroids alone, but it is estimated that this occurs in less than 20% of patients.2 Patients unresponsive to first-line therapy should undergo a diagnostic re-evaluation for a possible underlying disease, since AIHA associated with malignant tumors, ulcerative colitis, benign ovarian teratomas, or with IgM warm autoantibodies are often steroid-refractory.2
Second-line therapy
Once the decision for a second-line treatment has been taken, there are several options, although splenectomy and rituximab are the only second-line treatments with a proven short-term efficacy.2
Splenectomy
Splenectomy is commonly thought to be the most effective conventional second-line treatment of warm AIHA to be proposed to patients unresponsive or intolerant to corticosteroids, in those that require a daily maintenance dose of prednisone greater than 10 mg, and in those with multiple relapses.2 However, its efficacy has never been compared to that of other second-line approaches, and no convincing data on remission duration after surgery are available.1 Factors in favor of splenectomy as the best second-line therapy include its short-term efficacy and the good initial response rate: a partial or complete remission is obtained in approximately 2 in 3 patients (38–82% depending on the percentage of secondary cases which seem to be less responsive than idiopathic forms21). Moreover, a substantial number of them remain in remission for years without medication, with a presumed cure rate of up to 20%.2,22,23 It is worth mentioning that patients with persistent or recurrent hemolysis after splenectomy often require lower doses of corticosteroids than before surgery.2 A drawback of splenectomy is the lack of reliable predictors of the outcome, since its effectiveness is not related to disease duration, response to steroids nor the extent of splenic sequestration.24 Moreover, splenectomy may be associated with surgical complications (pulmonary embolism, intra-abdominal bleeding, abdominal abscess, abdominal wall hematoma), although laparoscopic intervention has lowered the surgical risk compared to conventional surgery (0.5–1.6% vs. 6%).25 The most feared complication after splenectomy is overwhelming sepsis due to encapsulated bacteria, with a risk of 3.3–5% and a mortality rate of up to 50%,26,27 even after the introduction of pre-operative vaccination against pneumococci, meningococci, and hemophilus. The role and efficacy of antibiotic prophylaxis in this setting remains unclear, and not all investigators recommend this approach.1,28 Finally, small, but not insignificant additional risks include thromboembolism and pulmonary hypertension.29,30 The rate of splenectomy in adults is not known2 while in a large pediatric series of 256 AIHA (99 of whom with Evans syndrome) splenectomy was performed in 13.9% of cases.5 It should be remembered that in spite of the fact that the incidence of infection in children and adults is reported to be similar, the death rates among children are higher than adults (1.7% vs. 1.3%).26
Rituximab
Rituximab, a monoclonal antibody directed against the CD20 antigen expressed on B cells, has been shown to be effective in AIHA, although the comparison of response rates in various studies is difficult in the absence of common response criteria. Recent reviews31,32 reported that rituximab (375 mg/m2 weekly for a median of 4 weeks) is effective in treating both warm AIHA and CAD, with a median response rate higher in the warm forms (overall response (OR) 83–87%, complete response (CR) 54–60% vs. OR 58%, CR 4.5%); disease free survival has been reported to be 72% at one and 56% at two years.33 Rituximab has been shown to be effective both in idiopathic and secondary AIHA, including those associated with autoimmune and lymphoproliferative disorders, and bone marrow transplant.31,32,34–37 Responses to treatment were observed in monotherapy or in combination with corticosteroids, immunosuppressants and interferon-α,35,36 and regardless of prior therapy.34,35 The time to response varies considerably, with some patients responding very quickly and others taking weeks or even months to achieve their maximum response.35,38 In a recent multicenter retrospective study, the time to response was one month post-initiation of rituximab in 87.5% and three months in 12.5% of patients.39 It is worth remembering that rituximab re-treatment may be effective35,39,40 and some patients responded to re-treatment more than once.34,35 Rituximab has also been found to be effective in Evans syndrome with a reported overall response of 83% (66% complete).41 The response is even greater (up to 94%) considering the more recent and numerous series.32 The treatment is effective also in children42 and in Evans syndrome secondary to lymphoproliferative or other autoimmune diseases.43,44 Rituximab treatment is well tolerated and no adverse events are reported for most patients, excluding infusion-related side effects.35,40,45 The drug has a well-established safety profile (infectious events in approx. 7%), although rare cases of progressive multifocal encephalopathy, mostly in onco-hematologic conditions, hepatitis B reactivation and other viral infections have been reported.31,32 To prevent hepatitis B reactivation both after rituximab and prolonged steroid therapy, antiviral prophylaxis is now recommended.46
In an attempt to minimize side-effects and reduce costs, low-dose rituximab (100 mg fixed dose/weekly for 4 weeks) was reported to be effective in patients with AIHA who failed to respond to conventional treatment, as monotherapy47 or in combination with alemtuzumab.13 Moreover, low-dose rituximab as first- or second-line therapy was able to induce an overall response rate of 89% (complete response 67%),14 and 68% relapse-free survival at 36 months,15 suggesting that this drug should be used early in the treatment scenario of AIHA. Finally, a recent phase III randomized trial showed that approximately 70% of patients treated with glucocorticoids and rituximab were still in remission at 36 months, compared with approximately 45% of those treated with steroids alone.10
Immunosuppressive drugs
Before the introduction of rituximab in the therapy of AIHA, azathioprine (100–150 mg/day) and cyclophosphamide (100 mg/day) were often used as second-line treatment because ‘good’ responses (40–60% of cases) had been reported in the early literature (although a subsequent critical analysis demonstrated that a response had been obtained in less than one-third of patients).1,2 Cyclosporin A has been used successfully in a limited number of refractory AIHA patients.1,22 In particular, long-term therapy with cyclosporine was reported to induce complete remission in 3 in 4 of warm AIHA patients with life-threatening hemolysis unresponsive to previous treatments.48 In association with prednisone and danazol, cyclosporin was shown to improve the complete response rate in 18 warm AIHA patients compared with 26 patients treated with only prednisone and danazol (89% vs. 58%), and to reduce the incidence of relapse.49,50 Only limited data on the use of mycophenolate mofetil in patients with refractory warm AIHA are available. Complete remission and good partial responses have been reported in all treated adult patients (9 idiopathic and 2 secondary to systemic lupus erythematosus).51–54 The drug has been proven to be effective in refractory immune cytopenias (9 AIHA) in children with the autoimmune lymphoproliferative syndrome, of whom 12 of 13 patients responded with reduction in doses or cessation of other immunosuppressive drugs;55 the treatment was well tolerated in all patients. It has been suggested that this drug could be included in the treatment arsenal of refractory immune cytopenias, as a steroid-sparing option.23 Recently, mycophenolate mofetil has also been successfully used in association with rituximab in a case of post-hematopoietic stem cell transplant, refractory AIHA.56
Other options
Danazol, a synthetic anabolic steroid with mild androgenic properties, has been successfully used in 28 AIHA patients concurrent with or after steroids, but its effectiveness was limited in refractory or relapsed cases, of whom only 43% achieved a complete remission.57 In another series of 17 patients treated with danazol plus prednisone, an excellent response was obtained as first-line therapy (8 of 10 patients), whereas treatment was less effective (3 of 7) in relapsed or refractory patients.58 In contrast, a more recent retrospective study did not observe any substantial modification in the response rate nor in the duration of prednisone therapy in patients treated with danazol.59 No article supporting its use has been published in the last decade.
Intravenous immunoglobulins (IVIG) are frequently used in AIHA, alone or in combination with prednisone,60 and mostly in children, probably because of their proven effectiveness in primary immune thrombocytopenia, and the relatively low incidence of adverse effects compared with other treatment options. However, their use is controversial, primarily because only small case series have been reported.1,22 A good response was obtained in 5 patients with recurrent warm AIHA associated with CLL,61 the recovery of the hemoglobin levels being faster when prednisone and high-dose IVIG were combined. In a retrospective study of 73 patients,62 a response was observed in 40% of cases, only 15% achieving hemoglobin levels of 10 g/dL or greater; children were more likely to respond (54%). In a recent guideline, high-dose immunoglobulin was not recommended for use in AIHA, except under certain life-threatening circumstances.63
Plasma exchange has been performed in a relatively small number of severely affected warm AIHA patients, both children and adults, in whom the anemia could not be stabilized with steroids and transfusion therapy alone, as a temporizing measure.1 The results were inconsistent, and favorable effects generally short-lived. Moreover, concomitant therapy with steroids and immunosuppressive drugs often made it difficult to define the contribution of this procedure to the outcome. McLeod et al.64 reviewed 17 cases of warm AIHA treated with plasma exchange showing that it seemed to stabilize the disease and increase the efficiency of blood transfusions in cases with fulminant hemolysis, whereas other acutely ill patients showed no improvement. A retrospective single center case-control study failed to demonstrate that plasma exchange increases red blood cell transfusion efficiency in severe autoimmune hemolytic anemia.65 In a summary of current indication categories endorsed by the American Association of Blood Banks (AABB) and the American Society for Apheresis, plasma exchange for AIHA is considered as a category III indication, i.e. an application representing “heroic or last-ditch efforts on behalf of a patient”.66
“Last option” treatments
High-dose cyclophosphamide (50 mg/kg/day for 4 days) followed by granulocyte colony-stimulating factor was effective in achieving complete remission in 5 of 8 patients with highly refractory warm AIHA.67
Alemtuzumab, a humanized anti-CD52 monoclonal antibody, has been shown to be effective in small series of patients with idiopathic refractory AIHA, with an overall complete remission rate in 13 of 16, including 3 pediatric cases.23,68,69 However, because of the high toxicity, it is considered a “last resort” option in severe idiopathic AIHA unresponsive to all previous treatments.2 Alemtuzumab induced an overall response in 11 of 12 cases with CLL-associated AIHA, refractory to corticosteroid, splenectomy and rituximab, suggesting that it should be considered even before rituximab in warm AIHA accompanied by progressive CLL.22,70–72 Ofatumumab, a monoclonal antibody targeting a unique epitope on CD20 that differs from that targeted by rituximab, has recently been successfully used in a case of CLL-associated warm AIHA refractory to rituximab.73
Hematopoietic stem cell transplantation
Information on the use of hematopoietic stem cell transplantation (HSCT) in warm AIHA is limited to single cases or small series, mostly Evans syndromes,1,74,75 with an overall complete remission rate of approximately 60% in allogeneic and 50% in autologous HSCT. The analysis of data of 36 patients with refractory cytopenias (n=7 AIHA, n=7 Evans syndrome) included in the Registry of the European Group of Blood and Marrow Transplantation showed a continuous remission in 1 of 7 autologous HSCT and 3 of 7 allogeneic HSCT, with a transplant-related mortality (TRM) of approximately 15%.75,76
Supportive therapy
Patients with AIHA may often require red blood cell (RBC) transfusion to maintain clinically acceptable hemoglobin values, at least until specific treatments become effective. The decision to transfuse should depend not only on the hemoglobin level, but rather on the patient’s clinical status and comorbidities (particularly ischemic heart or severe pulmonary disease), the acuteness of disease at onset, the rapidity of progression of the anemia, and the presence of hemoglobinuria or hemoglobinemia and other manifestations of severe hemolysis.1 The blood transfusion should never be denied to patients in a critical clinical situation, even in cases in which no truly compatible units can be found, since warm autoantibodies are frequently panreactive. ABO- and RhD-matched red cell concentrates can in any case be safely administered in urgent cases if alloantibodies (known to occur in 12–40% of AIHA patients1) are reasonably excluded on the basis of the previous transfusion and/or pregnancy history. In less urgent cases, an extended phenotyping is advisable and compatible red cell units may be selected for transfusion.77 In some patients, more complex procedures, such as warm autoadsorption or allogeneic adsorption, may be needed for the detection of alloantibodies.1 Undetected alloantibodies could be the cause of increased hemolysis following transfusion, which might falsely be attributable to an increase in the severity of AIHA.1 In case the autoantibody specificity is well-defined (most frequently within the Rh system), it is still debated whether it is preferable to ignore or to respect it in the selection of blood to be transfused, the latter approach implying the administration of RBCs containing Rh antigens that the patients lacks with the risk of alloimmunization. Some authors recommend ignoring the specificity of the autoantibody because it is not extensively proven that antigen-negative RBC transfusions result in an increased erythrocyte survival.78 Moreover, some data suggest that patients with AIHA have an increased propensity to develop RBC alloantibodies after transfusion.1 Ignoring the autoantibody specificity has been demonstrated to be safe and effective in a great number of transfusions.79,80 To minimize risks of febrile non-hemolytic reactions due to anti-leukocyte antibodies, nowadays leuko-depleted red cells are used in AIHA patients. As regards the volume to be transfused, it is worth remembering that overtransfusion should be avoided both for hemodynamic reasons (particularly in elderly patients), and for the occurrence of hemoglobinemia and hemoglobinuria, which might not be due to alloantibody-induced hemolysis, as generally thought, but rather to the increase of the total mass of RBCs available for destruction. Finally, not only should the amount of blood transfused be limited, but RBCs should also be administered slowly, when possible, not exceeding 1 mL/kg/h.1
It is worth mentioning that C1-esterase inhibitor might have potential as a safe therapy to control complement-induced RBC destruction in AIHA patients.81 Moreover, the administration of erythropoietin was successfully used in patients with therapy-refractory AIHA, particularly in the presence of reticulocytopenia.82
Therapy of cold AIHA
The decision to treat CAD should be reserved for patients with symptomatic anemia, transfusion dependence, and/or disabling circulatory symptoms. In fact, non-severe asymptomatic forms of CAD may require only protection against exposure to cold temperatures and occasional transfusion support in winter.1,83,84 Erythrocyte transfusions can safely be given in CAD, provided appropriate precautions are taken. In particular, the patient and the extremity chosen for infusion should be kept warm, and the use of an in-line blood warmer is recommended. Moreover, infusion of cold liquids and blood products with a high plasma content should be avoided.1,84,85 In a recent retrospective analysis of 89 patients, 40% received transfusions during their disease course and 82% received drug therapy.84
As regards first-line therapy, response to steroids has never been supported by systematic studies and is still controversial, being effective in a small fraction of cases (14–35%) and usually requiring unacceptably high doses in order to maintain the remission.1,83,84,86,87 Therefore, this treatment, although still widely used in the clinical practice, is now discouraged.
Concerning conventional cytotoxic immunosuppressive drugs, monotherapy with chlorambucil or cyclophosphamide has shown some beneficial effect in small series (16% of cases),1,87,88 whereas no convincing responses were observed in the few patients treated with azathioprine87,89 and interferon-α or low-dose cladribine.90 Splenectomy is usually ineffective1,84 due to the fact that clearance of C3b-opsonized erythrocytes primarily occurs in the liver, although it has occasionally been reported to be effective in rare cases of IgG-mediated CAD. Finally, erythropoietin, widely used in the USA but not so often in Western and Northern Europe, has no evidence-based proof of efficacy.84
The availability of rituximab in the last 10–15 years has substantially changed the therapy of CAD, since this drug is directed against the pathogenic B-cell clone, which is detected in the majority of patients by flow cytometry and/or immunohistochemistry.84,87,91 The standard dosage was reported effective in approximately 60% of cases, with a response duration of one year, both in several case reports and in larger, prospective, but uncontrolled published trials.11,12,31,32 The median time to response was 1–2 months and responses were observed following a second, and even a third course, in relapsed cases. Rituximab is now recommended as the first-line treatment of CAD,84 although complete and sustained remissions are uncommon.91 Furthermore, combined treatment with rituximab and fludarabine administered orally (40 mg/m2 on Days 1–5) resulted in higher response rates (76% of cases) and sustained remissions (estimated median response duration 6.5 years).92 Since hematologic toxicities and infective complications were common, this regimen is suggested for cases refractory to 1–2 courses of rituximab.84 In a more recent retrospective analysis of 89 patients, rituximab was associated with response rates of approximately 80% (both as single-agent and in combination therapy), a longer response duration (median 2 years), and a lower proportion of patients needing further treatment (55%).91 Finally, plasmapheresis may be useful in acute hemolytic crisis and before surgery requiring hypothermia,93,94 although its effect is transient.
As regards new experimental approaches, improvement of anemia has been observed in 2 patients following monotherapy with bortezomib, an inhibitor of 26S proteasome,95 and in 2 cases after administration of eculizumab, the monoclonal anti-C5 antibody licensed for paroxysmal nocturnal hemoglobinuria.96,97 However, these observations need to be confirmed in prospective trials.
There is no evidence-based therapy for CAD secondary to malignant or infectious diseases. Generally, treatment of the underlying disease is accompanied by resolution of the hemolysis, particularly in lymphoproliferative diseases and Mycoplasma pneumonia.1,85 The association of corticosteroid therapy is a subject of debate, particularly in CAD secondary to infection.1,13,85 Its use is suggested in severe forms or in cases in which there is no spontaneous improvement within a few days.
Paroxysmal cold hemoglobinuria (PCH) is characterized by acute intravascular hemolysis mediated by the Donath-Landsteiner biphasic hemolysin, which binds to erythrocytes at low temperatures and causes complement-mediated hemolysis at 37°C. Most antibodies are IgG and directed against the P blood group system. In the past, PCH was mainly associated with syphilis, and now usually follows viral and bacterial infections, including Mycoplasma pneumonia.1 PCH is usually a self-resolving disease, although deaths have been reported.98 The few severe cases may require transfusions and steroid treatment, whose effectiveness is difficult to evaluate because of the transient nature of the hemolysis.1 Eculizumab was reported ineffective in a case of steroid-refractory PCH with associated myeloma.99
Approximately 7–8% of autoimmune hemolytic anemias have serological findings characteristic of warm AIHA and CAD, and are, therefore, classified as mixed forms.1,100 Caution had been expressed about this diagnosis, as sometimes it is made on the basis of inadequate serological studies.101 Petz et al.1 reported that 35% of patients with WAIHA have cold agglutinins reactive at 20°C, which were, however, clinically insignificant in almost all cases (only 5% reacted at 37°C). Some authors suggest that patients with mixed AIHA have a more severe onset and more chronic course than patients with other categories of AIHA, although a comprehensive comparison of the clinical course in the different forms has not been made. We retrospectively studied 157 AIHA patients followed-up at our institution and found that the most severe cases (hemoglobin lower than 6 g/dL at onset) were mainly mixed and atypical forms (DAT-negative, warm IgM positive). These cases frequently showed reticulocytopenia, which may contribute to the clinical picture. Moreover, cases with severe onset were more often refractory to first-line therapy and required 3 or more lines of therapy.
Conclusions
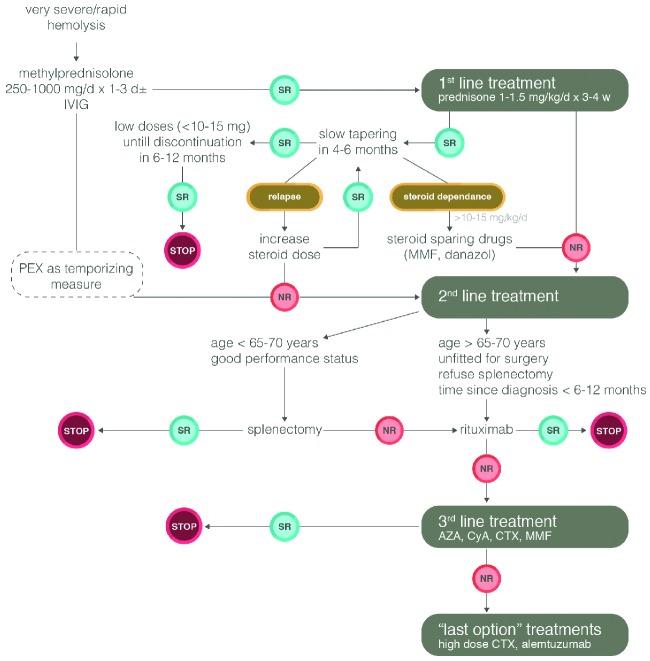
The therapeutic arsenal now available for steroid-refractory warm AIHA is certainly broader than in the past. However, no controlled clinical trials have yet been performed that can guide the choice of treatment.24 It is current opinion that the sequence of second-line therapy in primary warm AIHA should be splenectomy, rituximab, and thereafter any of the immunosuppressive drugs. Nevertheless, the choice of second-line therapies depends on the physician’s personal experience, the patient’s age and comorbidity, and patient preference.2,24 However, in clinical practice, rituximab is used with increasing frequency before splenectomy, particularly in the most severe cases and children aged under 5–6 years (www.AIEOP.org, Recommendations for the Management of AIHA in children). The therapeutic algorithm for warm AIHA adopted in our institution is shown in Figure 1. As experience with rituximab evolves, it is likely that this drug will be used earlier in therapy, before more toxic immunosuppressants, and in some cases in place of splenectomy. As regards CAD, rituximab is now recommended as first-line treatment.
Figure 1.
Treatment algorithm for warm AIHA in adults. SR: sustained response defined as maintenance of Hb values >10 g/dL over time; NR: no response; d: day; w: week; AZA: azathioprine; CyA: cyclosporine A; CTX: cyclophosphamide; MMF: mycophenolate mofetil; PEX: plasma exchange; IVIG: intravenous immunoglobulin.
Footnotes
This review article was originally published in the education book of the 19th congress of EHA (June 2014).
References
- 1.Petz LD, Garratty G. Immune Hemolytic Anemias. 2nd ed Philadelphia: Churchill Livingstone; 2004
- 2.Lechner K, Jager U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;16:1831–8
- 3.Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolytic anaemias in adults: a clinical review. Wien Klin Wochenschr. 2008;120:136–51
- 4.Silberstein LE, Cunningham MJ. Autoimmune Hemolytic Anemias. In: Hillyer CD, Silberstein LE, Ness PM, Anderson KC, Roback JD. (eds). Blood Banking and Transfusion Medicine. Basic Principles and Practice. 2nd ed Philadelphia: Churchill Livingstone; 2007
- 5.Aladjidi N, Leverger G, Leblanc T, Picat MQ, Michel G, Bertrand Y, et al. New insights into childhood autoimmune hemolytic anemia: a French national observational study of 265 children. Haematologica. 2011;96:655–63
- 6.Arndt PA, Leger RM, Garratty G. Serologic findings in autoimmune hemolytic anemia associated with immunoglobulin M warm autoantibodies. Transfusion. 2009;49:235–42
- 7.Kamesaki T, Toyotsuji T, Kajii E. Characterization of direct antiglobulin test-negative autoimmune hemolytic anemia: a study of 154 cases. Am J Hematol. 2013;88:93–6
- 8.Liesveld JL, Rowe JM, Lichtman MA. Variability of the erythrocyte response in autoimmune hemolytic anemias: analysis of 109 cases. Blood. 1987;69:820–6
- 9.Conley CL, Lippman SM, Ness P. Autoimmune hemolytic anemia with reticulocytopenia. A medical emergency. JAMA. 1980;244:1688–90
- 10.Birgens H, Frederiksen H, Hasselbalch HC, Rasmussen IH, Nielsen OJ, Kjeldsen L, et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163:393–9
- 11.Berentsen S, Ulvestad E, Gjertsen BT, Hjorth-Hansen H, Langholm R, Knutsen H, et al. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood. 2004;103:2925–8
- 12.Schöllkopf C, Kjeldsen L, Bjerrum OW, Mourits-Andersen HT, Nielsen JL, Christensen BE, et al. Rituximab in chronic cold agglutinin disease: a prospective study of 20 patients. Leuk Lymphoma. 2006;47:253–60
- 13.Gómez-Almaguer D, Solano-Genesta M, Tarín-Arzaga L, Herrera-Garza JL, Cantú-Rodríguez OG, Gutiérrez-Aguirre CH, et al. Low-dose rituximab and alemtuzumab combination therapy for patients with steroid-refractory autoimmune cytopenias. Blood. 2010;116:4783–5
- 14.Barcellini W, Zaja F, Zaninoni A, Imperiali FG, Battista ML, Di Bona E, et al. Low-dose rituximab in adult patients with idiopathic autoimmune hemolytic anemia: clinical efficacy and biological studies. Blood. 2012;119:3691–7
- 15.Barcellini W, Zaja F, Zaninoni A, Imperiali FG, Di Bona E, Fattizzo B, et al. Sustained response to low-dose rituximab in idiopathic autoimmune hemolytic anemia. Eur J Haematol. 2013;91:546–51
- 16.Naithani R, Agrawal N, Mahapatra M, Kumar R, Pati HP, Choudhry VP. Autoimmune hemolytic anemia in children. Pediatr Hematol Oncol. 2007;24:309–15
- 17.Gupta V, Shukla J, Bhatia BD. Autoimmune Hemolytic Anemia. Ind J Ped. 2008;75:451–4
- 18.Dussadee K, Taka O, Thedsawad A, Wanachiwanawin W. Incidence and risk factors of relapses in idiopathic autoimmune hemolytic anemia. J Med Assoc Thai. 2010;93(Suppl 1):S165–S170
- 19.Meyer O, Stahl D, Beckhove P, Huhn D, Salama A. Pulsed high-dose dexamethasone in chronic autoimmune haemolytic anaemia of warm type. Br J Haematol. 1997;98:860–2
- 20.Ozsoylu F. Megadose methylprednisolone for the treatment of patients with Evans syndrome. Pediatr Hematol Oncol. 2004;21:739–40
- 21.Akpek G, McAneny D, Weintraub L. Comparative response to splenectomy in Coombs-positive auto-immune hemolytic anemia with or without associated disease. Am J Hematol. 1999;61:98–102
- 22.Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transf Med Rev. 2010;24:195–210
- 23.Jaime-Pérez JC, Rodriguez-Martinez M, Gomez-de-Léon A, Tarin-Arzaga L, Gomez-Almaguer D. Current approaches for the treatment of autoimmune hemolytic anemia. Arch Immunol Ther Exp. 2013;61:385–95
- 24.Crowther M, Chan YL, Garbett IK, Lim W, Vickers MA, Crowther MA. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011;118:4036–40
- 25.Casaccia M, Torelli P, Squarcia S, Sormani MP, Savelli A, Troilo BM, et al. Laparoscopic splenectomy for hematologic diseases: a preliminary analysis performed on the Italian Registry of Laparoscopic Surgery of the Spleen (IRLSS). Surg Endosc. 2006;20:1214–20
- 26.Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43:182–6
- 27.Davidson RN, Wall RA. Prevention and management of infections in patients without a spleen. Clin Microbiol Infect. 2001;7:657–60
- 28.Newland A, Provan D, Myint S. Preventing severe infection after splenectomy. BMJ. 2005;331:417–8
- 29.Krauth MT, Lechner K, Neugebauer EA, Pabinger I. The post-operative splenic/portal vein thrombosis after splenectomy and its prevention-an unresolved issue. Haematologica. 2008;93:1227–32
- 30.Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–81
- 31.Garvey B. Rituximab in the treatment of autoimmune haematological disorders. Br J Haematol. 2008;14:149–69
- 32.Barcellini W, Zanella A. Rituximab therapy for autoimmune haematological diseases. Eur J Intern Med. 2011;22:220–9
- 33.Dierickx D, Verhoef G, Van Hoof A, Mineur P, Roest A, Triffet A, et al. Rituximab in autoimmune haemolytic anaemia and immune thrombocytopenic purpura: a Belgian retrospective multicentric study. J Intern Med. 2009;266:484–91
- 34.Peñalver FJ, Alvarez-Larrán A, Díez-Martin JL, Gallur L, Jarque I, Caballero D, et al. Rituximab is an effective and safe therapeutic alternative in adults with refractory and severe autoimmune hemolytic anemia. Ann Hematol. 2010;89:1073–80 [DOI] [PubMed] [Google Scholar]
- 35.Zecca M, Nobili B, Ramenghi U, Perrotta S, Amendola G, Rosito P, et al. Rituximab for the treatment of refractory autoimmune hemolytic anemia in children. Blood. 2003;101:3857–61 [DOI] [PubMed] [Google Scholar]
- 36.Narat S, Gandla J, Hoffbrand AV, Hughes RG, Mehta AB. Rituximab in the treatment of refractory autoimmune cytopenias in adults. Haematologica. 2005;90:1273–4 [PubMed] [Google Scholar]
- 37.D’Arena G, Laurenti L, Capalbo S, D’Arco AM, De Filippi R, Marcacci G, et al. Rituximab therapy for chronic lymphocytic leukemia-associated autoimmune hemolytic anemia. Am J Hematol. 2006;81:598–602 [DOI] [PubMed] [Google Scholar]
- 38.Zaja F, Vianelli N, Sperotto A, Patriarca F, Tani M, Marin L, et al. Anti-CD20 therapy for chronic lymphocytic leukemia-associated autoimmune diseases. Leuk Lymphoma. 2003;44:1951–5 [DOI] [PubMed] [Google Scholar]
- 39.Maung SW, Leahy M, O’Leary HM, Khan I, Cahill MR, Gilligan O, et al. A multi-center retrospective study of rituximab use in the treatment of relapsed or resistant warm hemolytic anemia. Br J Haematol. 2013;163:118–22 [DOI] [PubMed] [Google Scholar]
- 40.Rao A, Kelly M, Musselman M, Ramadas J, Wilson D, Grossman W, et al. Safety, efficacy, and immune reconstitution after rituximab therapy in pediatric patients with chronic or refractory hematologic autoimmune cytopenias. Pediatr Blood Cancer. 2008;50:822–5 [DOI] [PubMed] [Google Scholar]
- 41.Norton A, Roberts I. Management of Evans syndrome. Br J Haematol. 2006;132:125–37 [DOI] [PubMed] [Google Scholar]
- 42.Bader-Meunier B, Aladjidi N, Bellmann F, Monpoux F, Nelken B, Robert A, et al. Rituximab therapy for childhood Evans syndrome. Haematologica. 2007;92:1691–4 [DOI] [PubMed] [Google Scholar]
- 43.Rodella E, Pacquola E, Bianchini E, Ramazzina E, Paolini R. Consolidation treatment with rituximab induces complete and persistent remission of mixed type Evans syndrome. Blood Coagul Fibrinolysis. 2008;19:315–8 [DOI] [PubMed] [Google Scholar]
- 44.Michel M, Chanet V, Dechartres A, Morin AS, Piette JC, Cirasino L, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009;114:3167–72 [DOI] [PubMed] [Google Scholar]
- 45.Bussone G, Ribeiro E, Dechartres A, Viallard JF, Bonnotte B, Fain O, et al. Efficacy and safety of rituximab in adults’ warm antibody autoimmune haemolytic anemia: retrospective analysis of 27 cases. Am J Hematol. 2009;84:153–7 [DOI] [PubMed] [Google Scholar]
- 46.Marzano A, Angelucci E, Andreone P, Brunetto M, Bruno R, Burra P, et al. Prophylaxis and treatment of hepatitis B in immunocompromised patients. Dig Liver Dis. 2007;39:397–408 [DOI] [PubMed] [Google Scholar]
- 47.Provan D, Butler T, Evangelista ML, Amadori S, Newland AC, Stasi R. Activity and safety profile of low-dose rituximab for the treatment of autoimmune cytopenias in adults. Haematologica. 2007;92:1695–8 [DOI] [PubMed]